UT + Diagnostics Influenza A&B Antigen Rapid Test
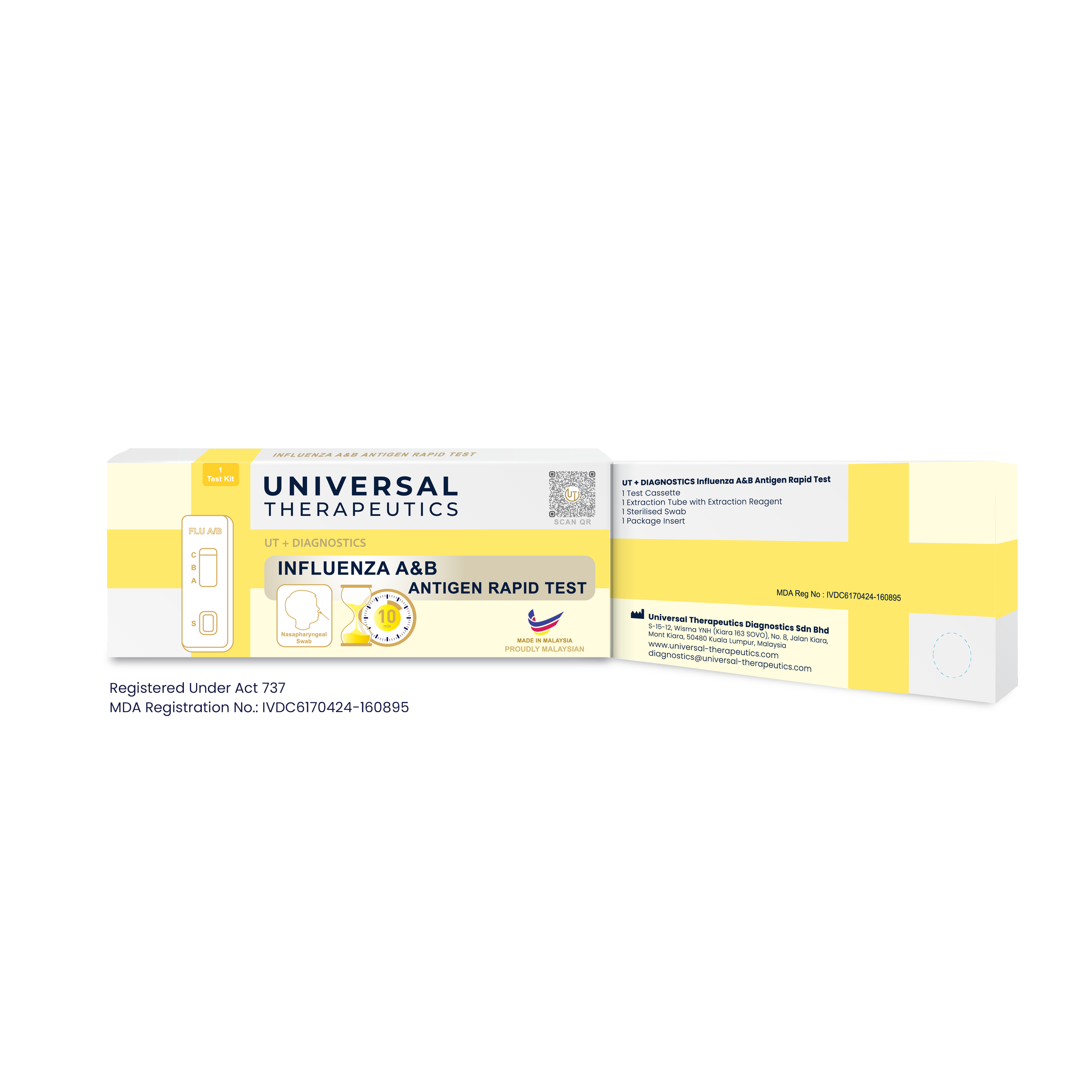
The UT + Diagnostics Influenza A&B Antigen Rapid Test is a chromatographic assay used for qualitative detection of influenza virus type A and B nucleoprotein antigens in human nasopharyngeal swab specimens.
This device is meant for professional use.
ISO 13485:2016 QMS certification
MDA Approved IVDC6170424-160895
Made in Malaysia
The UT + Diagnostics Influenza A&B Antigen Rapid Test is a chromatographic assay used for qualitative detection of influenza virus type A and B nucleoprotein antigens in human nasopharyngeal swab specimens.
This device is meant for professional use.
ISO 13485:2016 QMS certification
MDA Approved IVDC6170424-160895
Made in Malaysia
The UT + Diagnostics Influenza A&B Antigen Rapid Test is a chromatographic assay used for qualitative detection of influenza virus type A and B nucleoprotein antigens in human nasopharyngeal swab specimens.
This device is meant for professional use.
ISO 13485:2016 QMS certification
MDA Approved IVDC6170424-160895
Made in Malaysia
BENEFITS
High Accuracy
The UT + Diagnostics Influenza A&B Antigen Rapid Test is highly specific (>99%) and sensitive (>99%), allowing accurate diagnosis. (1)
Easy, Clear, Rapid result
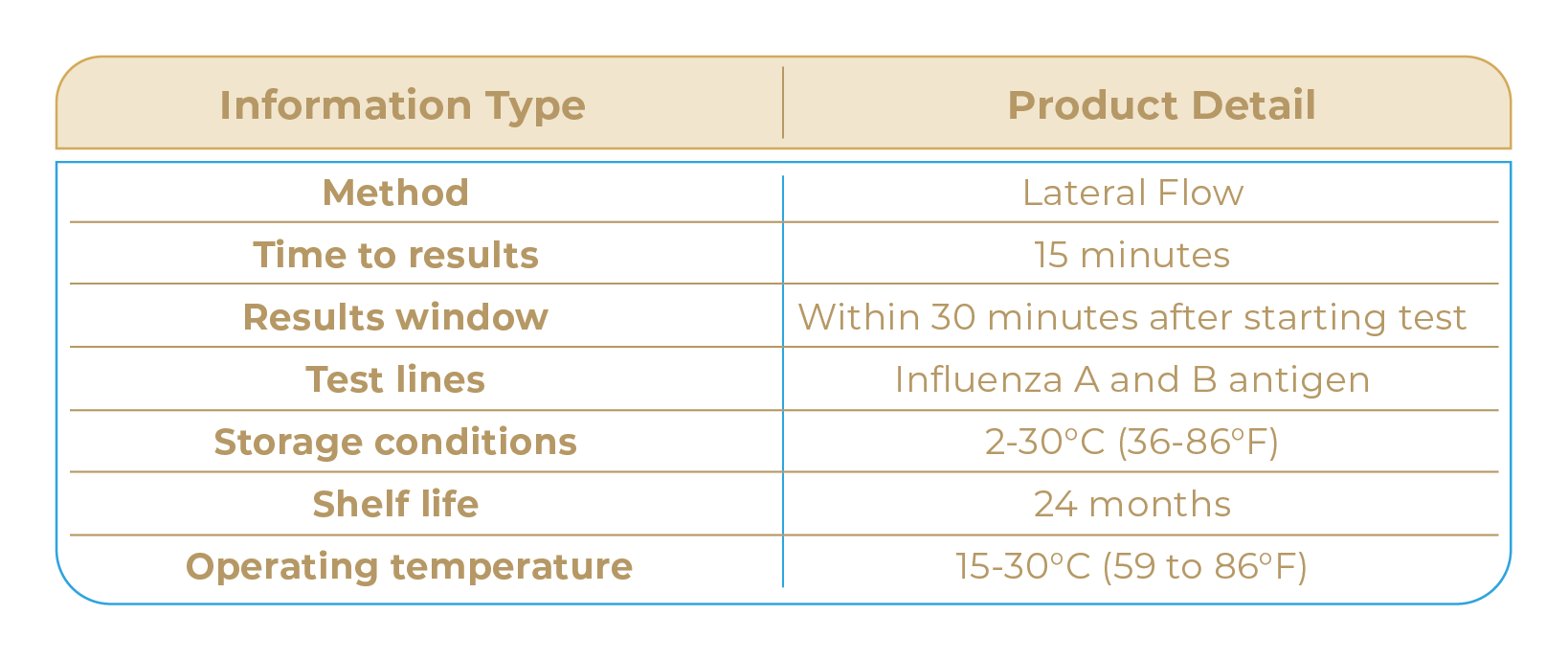
The UT + Diagnostics Influenza A&B Antigen Rapid Test detects the presence or absence of Influenza A and B antigens using nasopharyngeal swab samples in 15 minutes. No equipment is required to process the specimens or read the results.
Bulk Testing
The UT + Diagnostics Influenza A&B Antigen Rapid Test is scalable and suitable for bulk testing, making it suitable to be used in a variety of healthcare settings, including hospitals, clinics, schools, and workplaces.
Cost-Effective
The UT + Diagnostics Influenza A&B Antigen Rapid Test can help reduce healthcare costs by streamlining the diagnostic process, reducing the number of days in hospital, and the use of antibiotics.
PERFORMANCE
The UT + Diagnostics Influenza A&B Antigen Rapid Test is sensitive enough to detect patients with a high viral load within 3-4 days of illness onset.
SPECIFICATIONS
CONTENTS
1 test device in individual foil pouch
1 extraction tube with extraction buffer
1 sterilised swab
1 package insert
REFERENCES:
1. Universal Therapeutics Diagnostics Sdn Bhd (2023). Instructions For Use: UT + Diagnostics Influenza A&B Antigen Rapid Test.
2. <same reference>