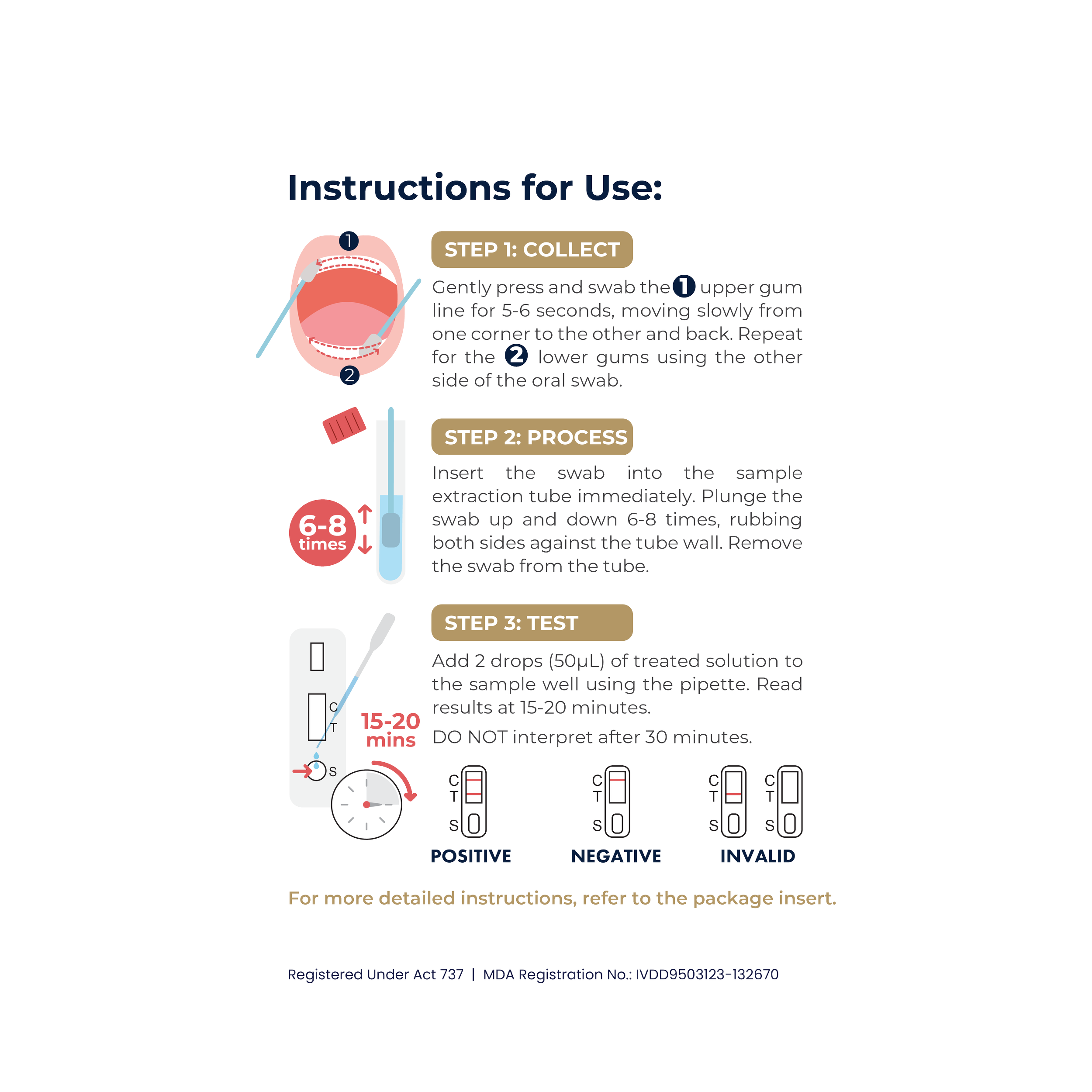
AwarerPRO HIV 1/2 Rapid Test (Oral Fluid)
THIS IS A PROFESSIONAL KIT. FOR PURCHASE AND PRICING, PLEASE CONTACT US DIRECTLY.
The AwarerPRO HIV 1/2 Rapid Test (Oral Fluid) is a single use, rapid chromatographic immunoassay for the qualitative detection of antibody to Human Immunodeficiency Virus (HIV) type-1 and/or type-2 in human oral mucosal exudate. It is intended for use in medical institutions and self-testing by lay users as an aid for the diagnosis and management of patients related to infection with HIV.
ISO 13485:2016 QMS certification
MDA approved IVDD9503123-132670
Made in Malaysia
THIS IS A PROFESSIONAL KIT. FOR PURCHASE AND PRICING, PLEASE CONTACT US DIRECTLY.
The AwarerPRO HIV 1/2 Rapid Test (Oral Fluid) is a single use, rapid chromatographic immunoassay for the qualitative detection of antibody to Human Immunodeficiency Virus (HIV) type-1 and/or type-2 in human oral mucosal exudate. It is intended for use in medical institutions and self-testing by lay users as an aid for the diagnosis and management of patients related to infection with HIV.
ISO 13485:2016 QMS certification
MDA approved IVDD9503123-132670
Made in Malaysia
THIS IS A PROFESSIONAL KIT. FOR PURCHASE AND PRICING, PLEASE CONTACT US DIRECTLY.
The AwarerPRO HIV 1/2 Rapid Test (Oral Fluid) is a single use, rapid chromatographic immunoassay for the qualitative detection of antibody to Human Immunodeficiency Virus (HIV) type-1 and/or type-2 in human oral mucosal exudate. It is intended for use in medical institutions and self-testing by lay users as an aid for the diagnosis and management of patients related to infection with HIV.
ISO 13485:2016 QMS certification
MDA approved IVDD9503123-132670
Made in Malaysia
EQUAL PERFORMANCE, GREATER CONVENIENCE
Reliable Results
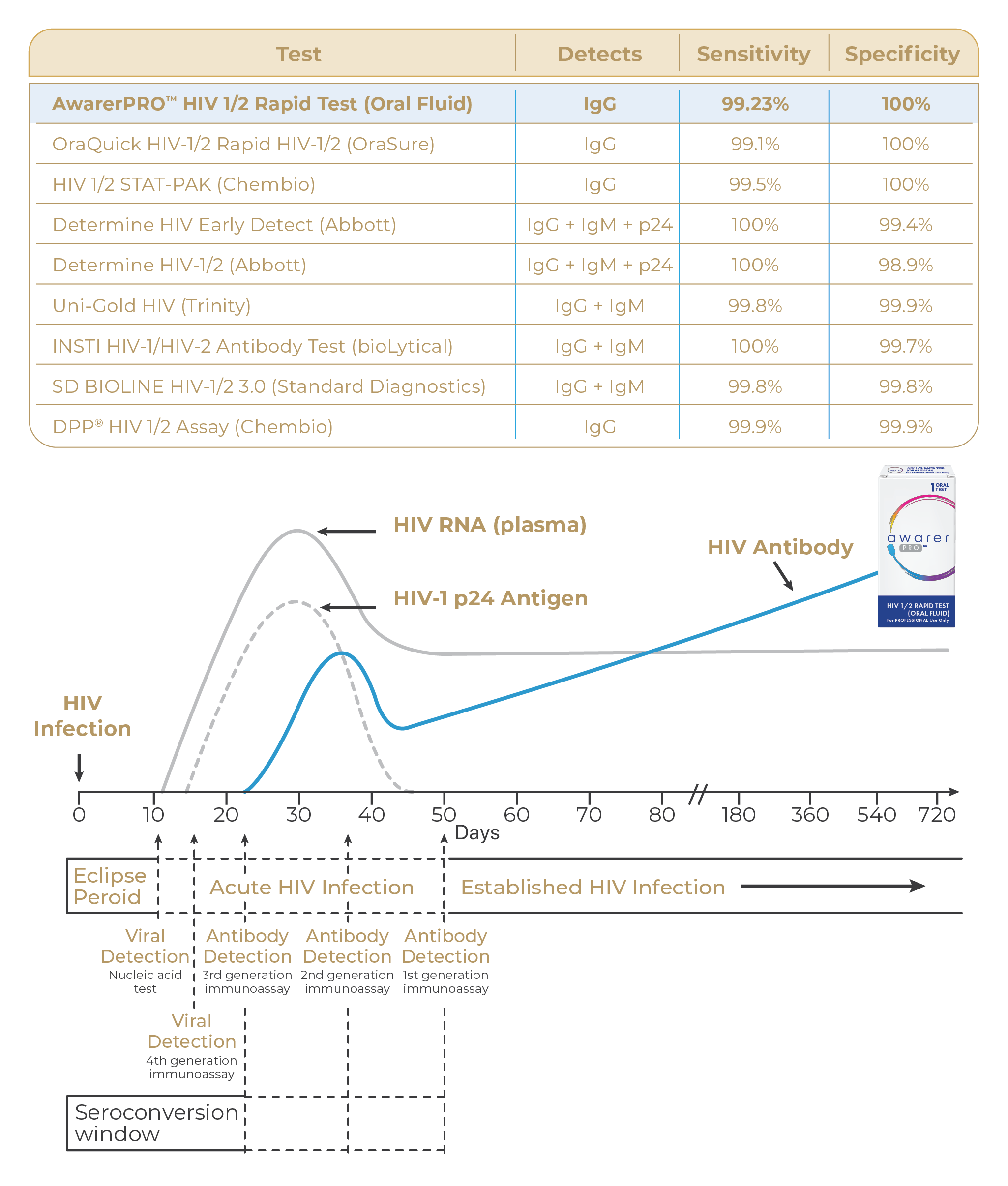
The AwarerPRO HIV 1/2 Rapid Test (Oral Fluid) has an impressive diagnostic sensitivity of 99.23%, and specificity of 100.00% with overall accuracy of 99.61%, making it a highly reliable method for HIV screening. (1)
Non-Invasive, Painless
The AwarerPRO HIV 1/2 Rapid Test (Oral Fluid) is non-invasive, requiring only a simple swab of the inside of the mouth. This makes it more comfortable for patients and reduces the fear associated with needles.
Confidential, Anonymous Testing
The AwarerPRO HIV 1/2 Rapid Test (Oral Fluid) can be used in a private setting, which encourages individuals to get tested without the fear of judgment or disclosure concerns.
No Mess or Contamination
AwarerPRO HIV 1/2 Rapid Test (Oral Fluid) eliminates the risk of mess or contamination associated with blood-based tests making it safer for both the person being tested and healthcare professionals.
Portable, Easy to Use
AwarerPRO HIV 1/2 Rapid Test (Oral Fluid) is easy to administer and doesn't require specialized medical training or equipment allowing for widespread testing in various healthcare settings.
Fast Results
AwarerPRO HIV 1/2 Rapid Test (Oral Fluid) provides quick results, within 15 minutes. This speed allows healthcare professionals to offer timely guidance and support to individuals.
PERFORMANCE ON PAR WITH LEADING HIV TEST KITS (2)
AwarerPRO HIV 1/2 Rapid Test (Oral Fluid) is a 3rd generation antibody tests with estimated median window period of 22 days where 13-46% chance of false negative result may occur. By 90 days, it able to detect more than 99% of the antibodies with 0-1% chance of false negative result. (3)
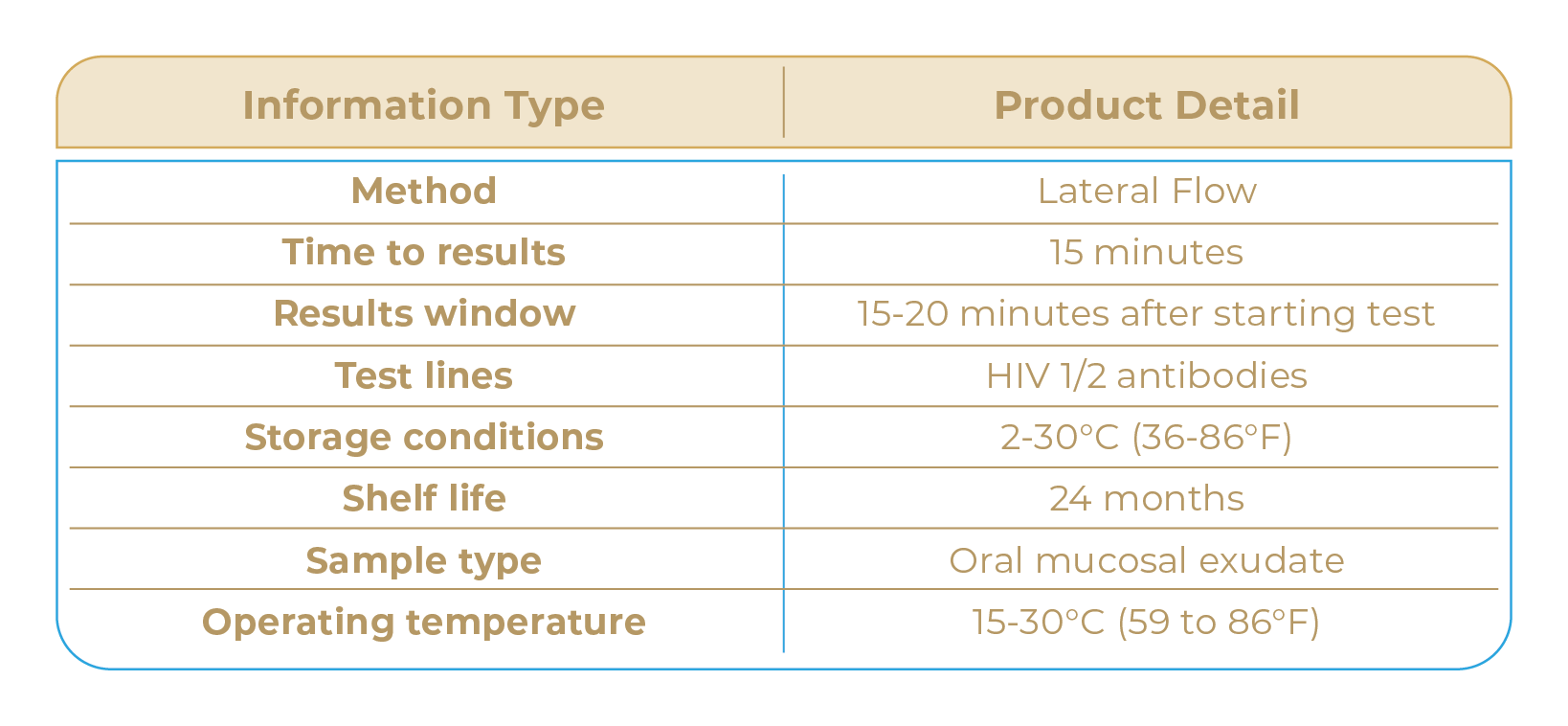
SPECIFICATIONS
CONTENTS
1 test cassette and pipette
1 sample extraction tube
1 oral swab
1 biohazard bag
1 package insert (instructions for use)
REFERENCES
1.Universal Therapeutics Sdn Bhd. (2023). Instructions for Use:AwarerPRO HIV 1/2 Rapid Test (Oral Fluid).
2.World Health Organization. HIV assays: laboratory performance and other operational characteristics: rapid diagnostic tests (combined detection of HIV-1/2 antibodies and discriminatory detection of HIV-1 and HIV-2 antibodies): report 18. 2015.
3.Taylor D, Durigon M, Davis H, et al. Probability of a false-negative HIV antibody test result during the window period: a tool for pre- and post-test counselling. Int J STD AIDS. 2015;26(4):215-224. doi:10.1177/0956462414542987.