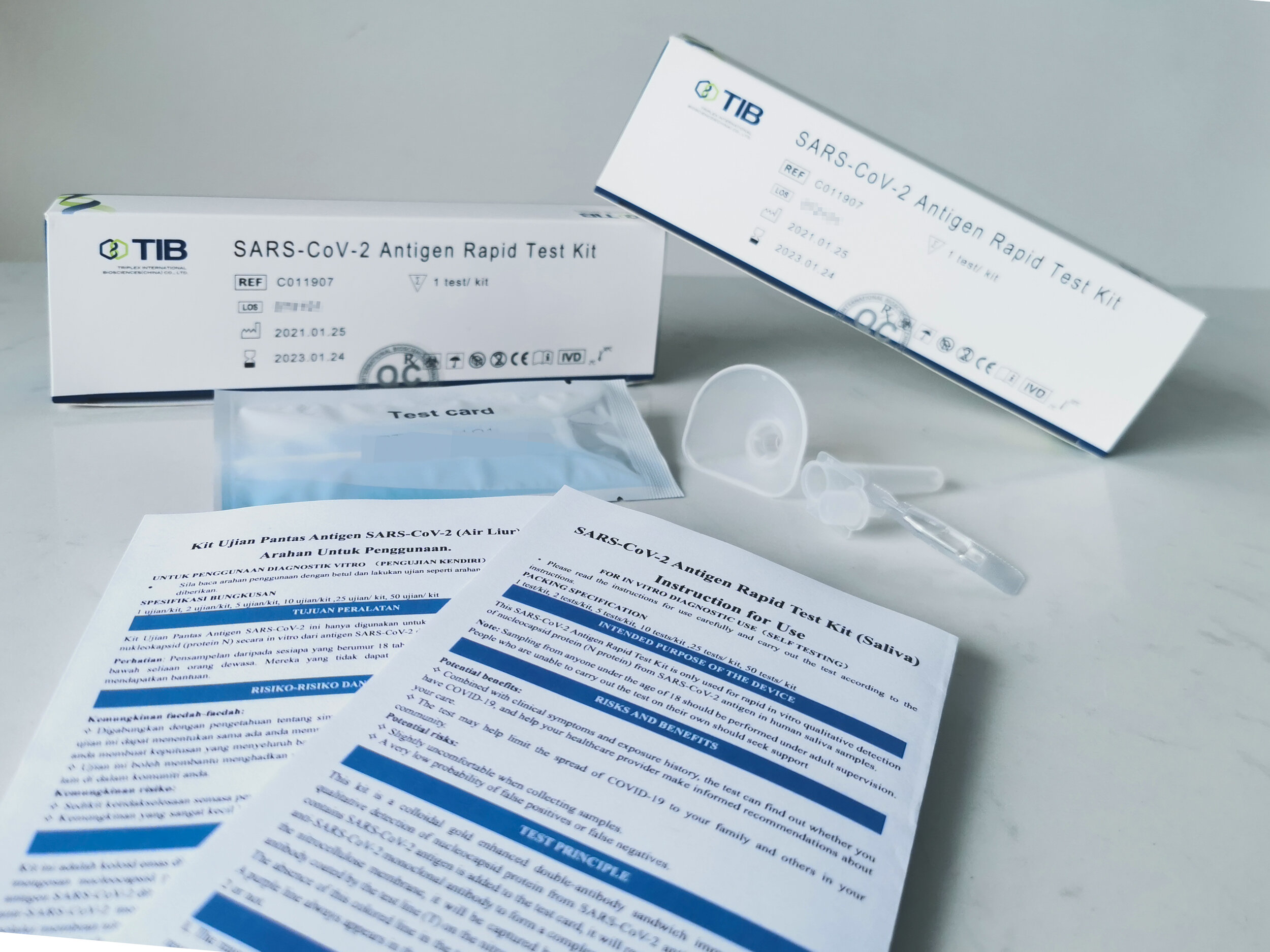
Medical Device Authority approves TIB SARS-CoV-2 Antigen Rapid Test Kit (Saliva) for Self-Test in Malaysia
FOR IMMEDIATE RELEASE
Kuala Lumpur, Malaysia, 5 October 2021 - Universal Therapeutics, a multinational pharmaceutical and biotech company based in Malaysia, today announced that the Medical Device Authority of Malaysia (MDA) has approved the SARS-CoV-2 Antigen Rapid Test Kit (Saliva) for self-testing.
Manufactured by Triplex International Biosciences (China), the easy-to-use Rapid Test Kit can quickly detect the possibility of a COVID-19 infection, with a sensitivity of 100% specificity of 100% and accuracy of 100%. The test kit works by detecting the presence of the coronavirus nucleocapsid protein in saliva samples. Infants under the age of two are not recommended to use this test kit.
“With the relentless spread of COVID-19, new variants and the huge strain on public healthcare systems, this easy-to-use saliva self-test kit meets the demand for a quick, affordable and accurate way to detect the virus, thereby aiding virus containment efforts,” said Sidney Soares, Chief Executive Officer, Universal Therapeutics.
The kit will be made available through Universal Therapeutics’ network of hospitals, medical facilities, medical institutions and pharmacies. Find out more from your local pharmacy today!
For more information, please visit https://www.universal-therapeutics.com
For MDA’s list of approved COVID-19 test kits, visit https://portal.mda.gov.my/announcement/631-self-test-covid-19-test-kit-for-conditional-approval-approved.html
About Universal Therapeutics:
Universal Therapeutics is a multinational pharmaceutical and biotech company based in Kuala Lumpur, Malaysia. The company’s businesses include manufacturing, distribution and white label production of pharmaceutical products, medical supplies, and PPEs in Asia and throughout the world.
Universal Therapeutics distributes Covid 19 Rapid Antigen tests to wholesalers, medical institutions, and government organisations. All our products comply with World Health Organization (WHO) guidelines and are accredited and approved by various health ministries as safe for human use.
For more information, please contact:
Samuel CHAI
Group Chief Commercial Officer
Office: +60 3 9213 0318
Email: GroupPR@Universal-Therapeutics.com
Website: www.Universal-Therapeutics.com